Ammonia – NH3
This page ammonia ( NH3 ) – hydrogen nitride also known as – provides information about: properties, health effects, detection tools and systems – ammonia detector – and suitable respiratory protective equipment (gas mask or K with gas filter powered air purifying respirator).
Ammonia (NH3) properties
Ammonia ( NH3 ) - or hydrogen nitride – is synthesized industrially from nitrogen (N) and hydrogen (H2) using a catalyst (Haber-Bosch process). This substance is an important element for producing fertilizer and is used in the refrigeration industry. When dissolved in water, ammonia Used to produce household products. It is also used in the textile industry and many other applications.
- CAS
- 7664-41-7
- TWA (8 hours)
- 10ppm
- STEL (15 minutes)
- 20ppm
- LEL
- 15,0 %
- IP
- 10.18 eV
- DENSITY/AIR
- 0,59
- FILTER/SCBA
- K
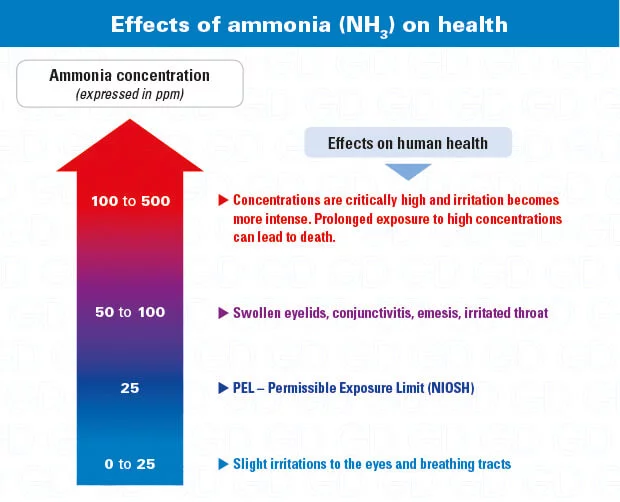
Health effects of ammonia
First of all, ammonia is poisonous (even in low concentrations) and is very harmful to the eyes and respiratory tract. is irritating . It can be easily detected by smell with its sharp and suffocating odor. This gas is also flammable And 15% to 28% by volume is explosive . Explosive reactions can occur when ammonia encounters other substances such as halogens, mercury or acetaldehyde.
Ammonia detector (NH3)
Even though NH3 can be detected via smell, only one ammonia detector can accurately measure concentrations of this gas. Two ammonia detection method is available: toxicity by ppm measurement or explosive potential by LEL percentage measurement (flammable gas detector).
For calibration and gas testing of your fixed or portable gas detectors ammonia calibration gas cylinders available.