Freons
This page contains basic information about freons, more commonly known as refrigerants or refrigerants, such as their technical specifications, health effects, how to detect them (freon detectors) and appropriate respiratory protection equipment.
Basic properties of Freon gases
Refrigerants were created to preserve food. The first ones used were carbon dioxide (CO2), sulfur dioxide (SO2), chloroethane (C2H5Cl), chloromethane (CH3Cl), ammonia (NH3) and some hydrocarbons.
There are many other refrigerants classified according to their properties:
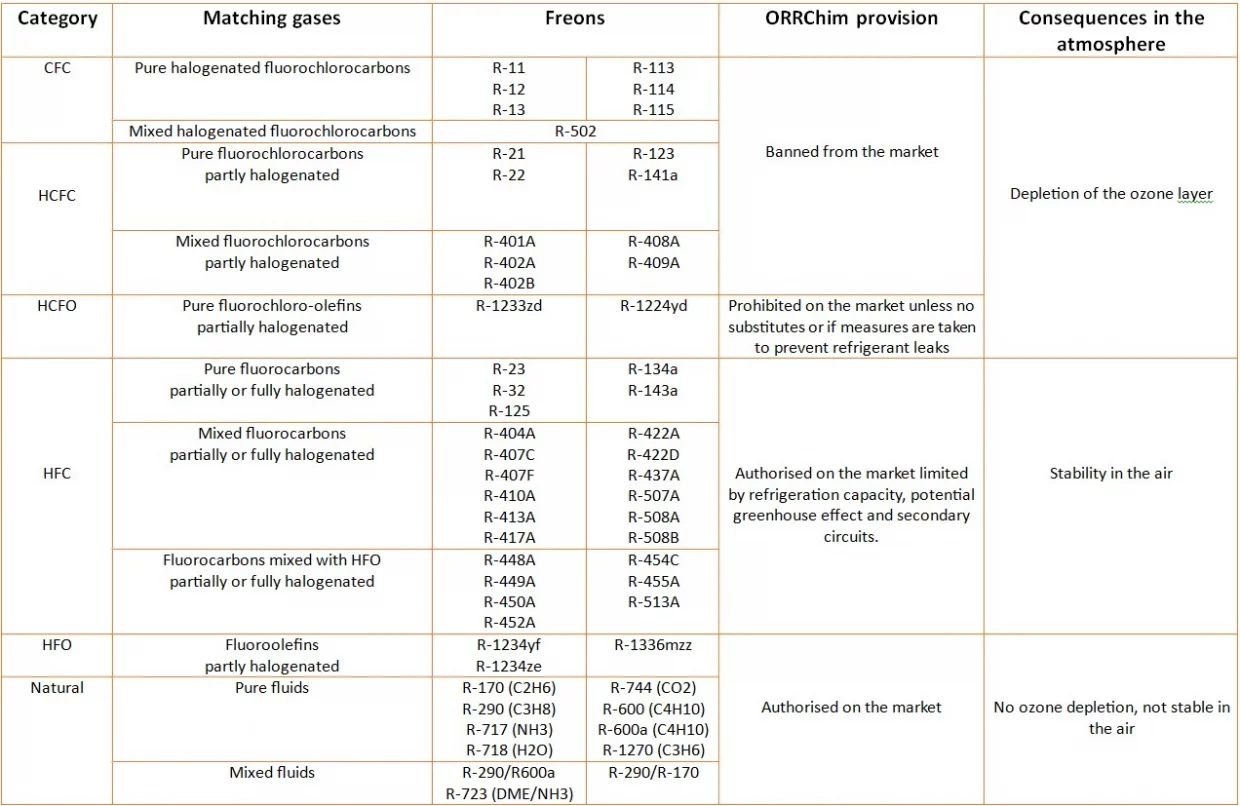
CFCs: Chlorofluorocarbons are halogenated fluorinated gases composed of carbon (CO), chlorine (Cl), and fluoride atoms (F).
HCFCs: Hydrochlorofluorocarbons are hydrogenated CFC gases that act as greenhouse gases to deplete the ozone layer. Therefore, it is prohibited to produce and use them.
HFCs: Hydrofluorocarbons are third-generation refrigerants that replaced freons, which were banned from the market. They are more efficient, less harmful and have a better impact on the environment.
HFOs: Hydrofluoroolefins are fourth generation gases. They pollute very little, making them ideal for use in air conditioning and commercial refrigeration.
Among the natural gases with refrigerant properties, the most commonly used is ammonia. It is the preferred refrigerant gas in industrial cooling systems, large pump systems, the food industry and high-power industrial coolers.
Effects of Freon on health
- Asphyxiation: Most freons, even CO2 natural gas, are asphyxiating gases. Their density weighs down the atmosphere and depletes the oxygen in the air.
- Toxicity: Refrigerants (such as ammonia) can cause cold burns, irritation, drowsiness, heart problems, etc. with prolonged exposure. why could it be.
- Explosives: Some refrigerants (such as propane – R290 or ethylene) are flammable and explosive when in contact with a combustion source.
Freon gas detector
Refrigerants that are toxic, explosive or asphyxiating pose a potential hazard to people and property in the event of a leak. Therefore, to optimize personal safety and avoid costs from refrigerant leaks, a It is essential to use a freon detection system.