Bromine – Br2
Bromine Here you can find basic information about (Br2): properties, health effects, detection devices and systems – bromine gas detector – and suitable respiratory protective equipment (gas mask or B with gas filter PAPR ).
Bromine (Br2) properties
Bromine ( Br2 ) is used instead of chlorine for bacterial management in wastewater treatment plants due to its disinfectant properties. Bromine is also used in agriculture to produce pesticides and fumigation agents. But above all, bromine is used in the pharmaceutical industry as a drug ingredient against pneumonia.
- CAS
- 7726-95-6
- TWA (8 hours)
- 0,1 ppm
- STEL (15 minutes)
- –
- LEL
- –
- IP
- 10.55 eV
- DENSITY/AIR
- 5.0
- FILTER/SCBA
- B
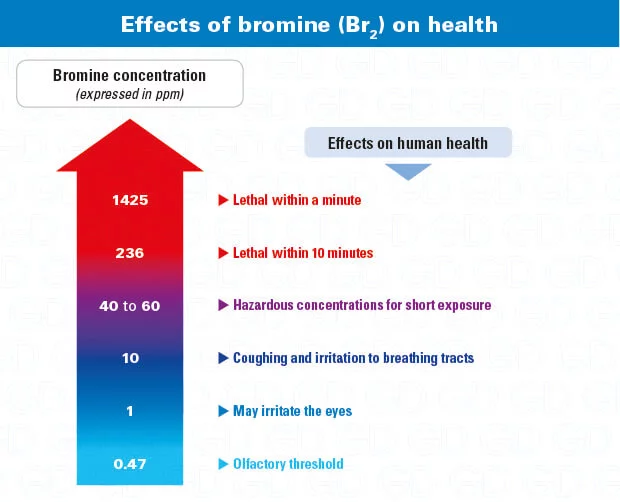
Health effects of bromine
At ambient temperature, bromine is a liquid with a brown-reddish stain and a pungent odor detectable below 1 ppm. When cold, by inhalation. Emits extremely toxic suffocating vapors (R26). Bromine is also essential for aquatic organisms. very corrosive and is toxic (R50) and can cause serious chemical burns (R35). On contact with water, it forms hydrobromic acid, a very corrosive substance.
Bromine gas detector (Br2)
Despite its pungent odor, it is only a bromine gas detector can accurately measure concentrations of this gas. To monitor bromine hazards, two Br2 detection technology available: either electrochemically with PortaSens (recommended) or with a photo-ionization lamp – bromine has an ionization capacity below 10.6 eV.
Br2 respiratory protection (Bromine)
Bromine irritates the eyes, so use only for short interventions. full face mask or A more comfortable one with type B gas filter powered air purifying respirator (PAPR) recommended. A self-contained breathing apparatus (SCBA) is mandatory when concentrations exceed 60 times the TLV.