Sulfuric acid – H2SO4
Here sulfuric acid ( H2SO4 ) or hydrogen sulfate You can find basic information about: properties, health effects, detection devices and systems – H2SO4 gas detector – and suitable respiratory protective equipment (gas mask or E-P2 with combined filter powered air purifying respirator).
Properties of sulfuric acid (H2SO4)
Sulfuric acid ( H2SO4 ) – hydrogen sulfate also known as – used to produce fertilizer and many chemical products (detergents, phosphoric acid). It is also largely used for pickling and chromating in metal processing. Finally, sulfuric acid is used both in the production of explosives and in batteries – particularly lead car batteries.
- CAS
- 7664-93-9
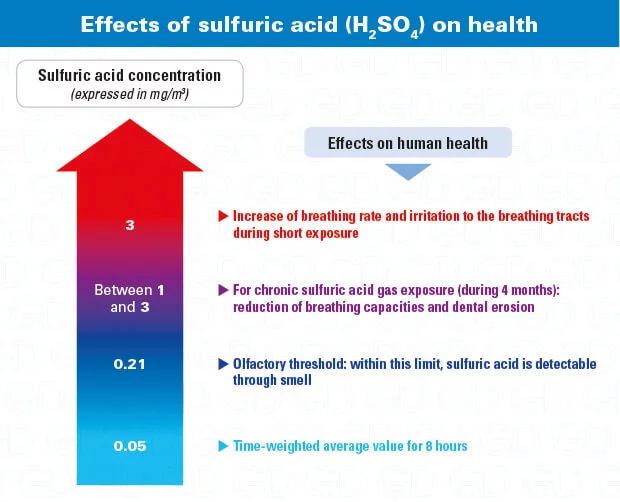
- TWA (8 hours)
- 0,2 ppm
- STEL (15 minutes)
- 0,5 ppm
- LEL
- –
- IP
- 12.0 eV
- DENSITY/AIR
- 1.84
- FILTER/SCBA
- E-P3
Health effects of sulfuric acid
At ambient temperature, sulfuric acid (H2SO4) is an oily, colorless and odorless (when pure gas) liquid. If dirty it may turn yellow-brown. This very corrosive substance skin burns And serious eye damage (H314) may cause . Sulfuric acid vapors are also very harmful and can be fatal if inhaled. When used alone, hydrogen sulfate is neither flammable nor explosive.
H2SO4 gas detector (sulfuric acid)
Given that this substance is harmful at very low concentrations, only one H2SO4 gas detector can accurately measure vapor concentrations. Sulfuric acid Only a few monitoring devices are available for their hazards: colorimetric gas detection tubes or very advanced and expensive systems.
Sulfuric acid respiratory protection (H2SO4)
Because sulfuric acid is very irritating to the eyes, exposed workers should avoid short-term applications. full face mask or with combined E-P3 filter a more comfortable powered air purifying respirator should prefer. If concentrations exceed 60 times the OEL, a self-contained breathing apparatus is required.